There has now been almost 40 years of experience in the U.S. of CES as a non medication treatment for anxiety. Also noteworthy is that among the more than 2000 patients who have been involved in CES studies in the U.S there has been no significant, negative side...
CES Ultra Blog
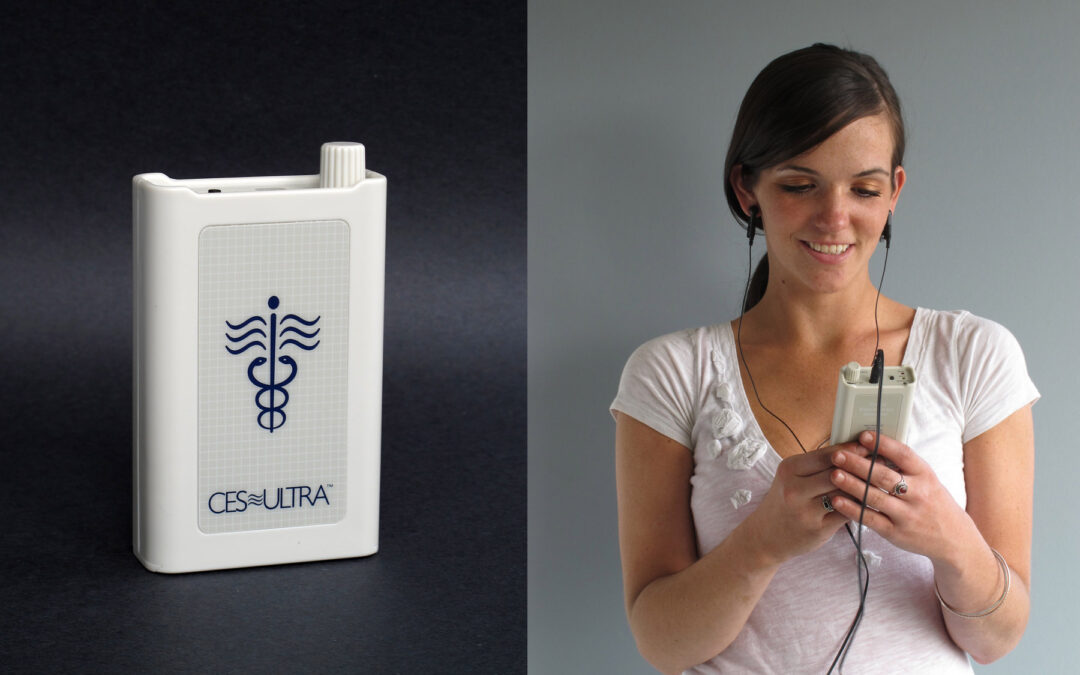
CES is a Non-Pharmacological Gentle Approach to the Treatment of Stress and Stress-Related Disorders
Cranial Electrotheapy Stimulation is a unique and viable bio-electric approach which enhances the homeostasis of the biological central nervous system—the tendency for intrinsic balance within a system. User-friendly, it employs mild battery-powered electrical...

CES Helps Anxiety and Sleep Disorder
Vignette #4 The patient is a fifty-two year old married female whose psychological problems took the form of depressive and anxious symptomology with an accompanying hyperkinetic element consisting of excessive energy that would not allow her sit still and experience...

Focus Factor Side Effects
Focus Factor® bottle, not equal to symbol, fresh fruits, vegetables and nuts Focus Factor is a memory booster that provides supplemental nutrition to help you feel sharper and more alert. The ingredients of Focus Factor are vitamin A, vitamin C, vitamin D, vitamin E,...

Endorphines, CES and Aging Process
Depleted supplies of "feel good" transmitters means it will be impossible for you to feel happy, upbeat, motivated or on track. You will feel just the opposite: A decrease in energy and interest, feelings of worthlessness and a pervasive sense of helplessness to...

Common Applications of CES (Cranial Electrotherapy Stimulation)
Granted these exceptions, CES proves effective in many applications. In the western culture depression and anxiety seem the most common psychological problems of normal people in normal life. In both cases, clinicians distinguish between reactive and obscure...
Cranial Electrotherapy Stimulation (CES) and Neurotransmitters
Neurotransmitters are the brain chemicals that communicate information throughout our brain and body. They relay signals between nerve cells, called “neurons.” The brain uses neurotransmitters to tell your heart to beat, your lungs to breathe, and your stomach to...
Kids on Drugs (Thanks to Parents and Doctors)
Part I: The Dangers Part II Parents and Doctors are often overwhelmed when having to deal with ADHD kids. They often look for a shortcut—prescription drugs. Common brand names include stimulants such as Ritalin, Concerta, Adderall, Metadate, Vyvanse, and Provigil. Use...
Kids on Drugs ( Thanks to Parents and Doctors) – Part 2
Part 2: How CES, the Drug Free Alternative, Can Make a Difference Part 1 One Parent's Experience CES Ultra Improves Sleep, Reduces Anxiety, Irritability, and Depression in 14-year-old Male We've been doing a trial with the CES Ultra the past week. The subject was DS*,...
Why Psychiatry Needs CES (Cranial Electrotherapy Stimulation)
Insomnia Many patients benefit from improving sleep hygiene and as a treatment for insomnia. Others may improve using a sleep phase changes or treating the underlying problem such as sleep apnea, medical conditions, alcohol abuse, etc. For many others, recent...
