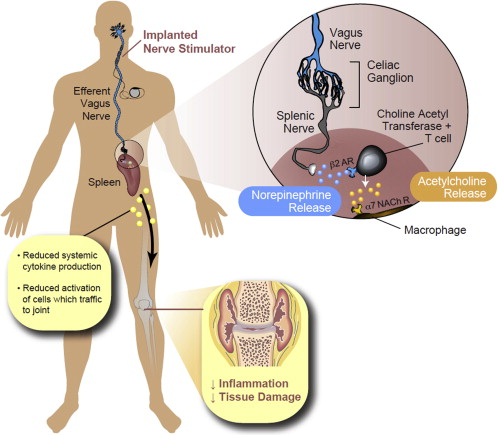
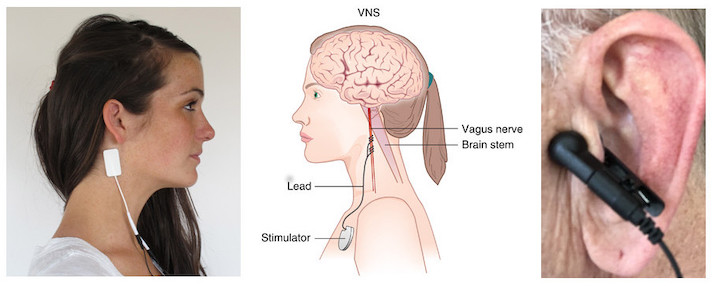
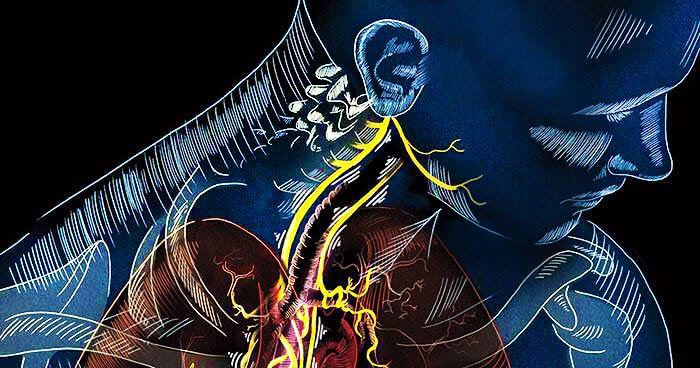
New Year’s Day marked a dispiriting milestone for one New Jersey woman: 20 months of symptoms of COVID-19. The woman, who asked for anonymity to protect her medical privacy, suffers from a variety of neurological problems that are associated with long COVID, including...