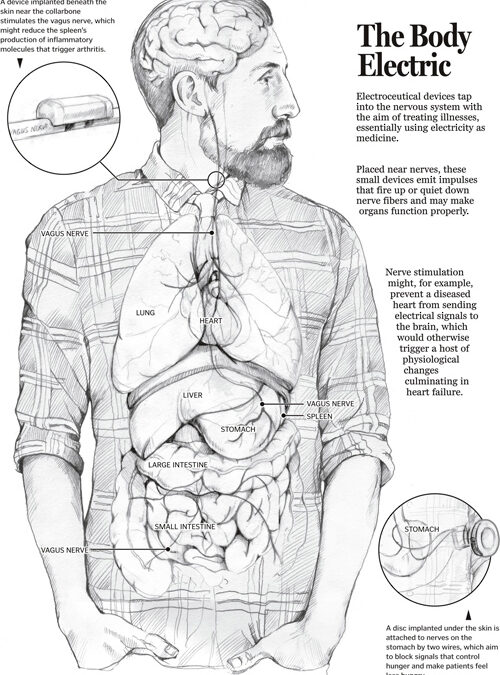
CES Research, CES Ultra device, Inflammation, Vagus Nerve Stimulation
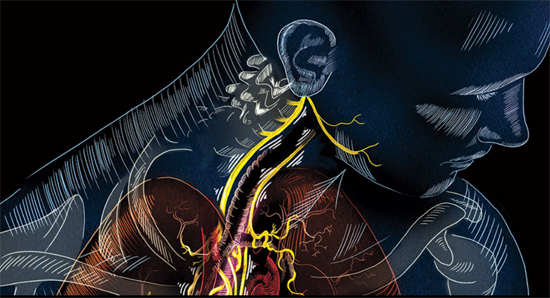
PART 1 PART 2 The CES ultra underscores its use of its conductive rubber ear clips. The rationale behind it is some interesting science on the ear; especially the vagus nerve and the special role it plays in the body. Read on. Ref: Wandering nerve could lead to range...
CES Research, CES Ultra device, Vagus Nerve Stimulation
PART 1 PART 2 The CES ultra underscores its use of its conductive rubber ear clips. The rationale behind it is some interesting science on the ear; especially the vagus nerve and the special role it plays in the body. Read on. Ref: Wandering nerve could lead to range...

CES Research, What they tell about CES Ultra
CES From the Early 1900s to 1953 and Beyond While electricity had been used in medicine for some time, in the early 1900s researchers in Europe began trying to find a way to use electricity to put people to sleep. They tried different pulse rates, various intensities...

CES Research, CES v. Drugs
The treatment seemed as ridiculous as wearing a foil hat to block CIA transmissions: 20 patients with overactive bladder syndrome had electrodes stuck to the soles of their feet for three hours every evening, producing a gentle vibration and causing the big toe to...